Enzymes: Isozymes and Regulation
Isozymes:
>Catalyze the same reaction but their physical and chemical properties exhibit significant differences.
>Physically distinct and separable (electrophoresis) forms of a given enzyme present in different cell types.
Nonfunctional Plasma Enzymes:
>Enzymes with no physiologic function in blood whether or not a substrate of the enzyme is present.
>Co-factors are lacking or too dilute.
>The enzyme concentration is far too low.
>The appearance of such enzymes in the plasma reflects destruction of cells from which these enzymes originate.
>This is based upon the assumption that organs contain a specific complement of enzymes and that when the organ is damaged cells in the organ lyse.
Principal Serum Enzymes Used in Clinical Diagnosis:
| Serum Enzyme | Major Diagnostic Use |
| Acid Phosphatase | Carcinoma of the prostate |
| Alanine Aminotransferase (ALT) | Hepatitis |
| Alkaline Phosphatase | Bone disorders; Obstructive liver disease |
| Asparate Aminotransferase (AST) | Myocardial infarction |
| Amylase | Acute pancreatitis |
| Ceruloplasmin | Wilson's disease |
| Creatine Phosphokinase | Myocardial infarction |
| Glutamyl Transpeptidase | Liver disease |
| Lactate Dehydrogenase (LDH) | Myocardial infarction |
| Troponin | Myocardial infarction |
| Lipase | Acute pancreatitis |
Knowledge about the location of different enzymes allows a physician to determine the source of disease.
Three Important Isozymes:
Creatine Phosphokinase (CPK): Creatine + ATP -----> Phosphocreatine + ADP
Tissue specific isozyme, dimer consisting of two subunits, M and B
MM isozyme - muscle
BB isozyme - brain
MB isozyme - heart
Lactate Dehydrogenase (LDH): Pyruvate + NADH -----> Lactate + NAD+
Tissue specific isozyme, tetramer consisting of two subunits, H and M
H4 isozyme - heart (aerobic, requires oxygen)
M4 isozyme - muscle (anaerobic, don't require oxygen)
Troponin: T and I are cardiac regulatory proteins that control the calcium mediated interaction between actin and myosin.
Amylase: Starch -----> "Glucose"
Tissue specific isozyme, monomer
Salivary- occurs in all other tissues.
Pancreatic- absence of this isozyme is indicative of pancreatic dysfunction.
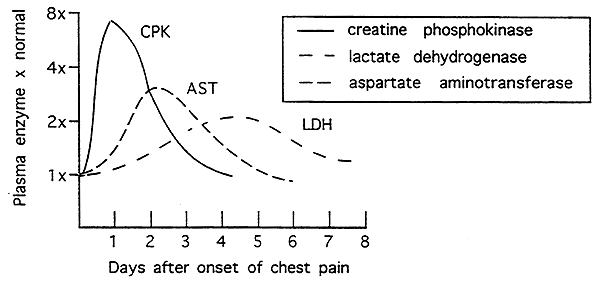
Clinical Correlate: Myocardial Infarction (MI):
Coronary arteries become clogged blocking blood flow to the heart (cardiac muscle).
Some cells do not survive this period of no blood flow.
Following a heart attack the cardiac cells lyse spilling their contents into the blood.
Creatine Phosphokinase (CPK)- MB isozyme appears w/in 24 hrs., normal w/in 48 hrs.
Lactate Dehydrogenase (LDH)- H4 isozyme may persist for two weeks
Aspartate Amino Transferase (AST)- peaks at 2 days, returns to normal by 4 days. (mitochondrial/cytoplasmic isozyme)
Cardiac Troponins (T and I)- appears first w/in 4-6 hrs., may be high for up to 2 weeks

Regulation of Enzyme Activity:
There are five primary forms of enzyme regulation: substrate availability, allosteric, post-translational modification, interaction with control proteins, zymogens.1. Substrate Availability:
Activity determined solely by the concentration (availability) of substrate.
2. Allosteric Regulation:
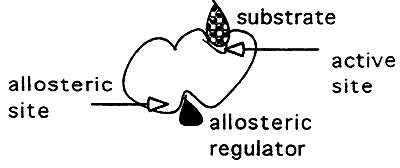
Binding of a non-competitive inhibitor.
Inhibitor binds to a site other than the active site -----> (-) cooperativity, this binding inhibits binding at the active site.
Active Enzyme- Relaxed state (high affinity for substrate)
Inactive Enzyme- Tense state (low affinity for substrate)
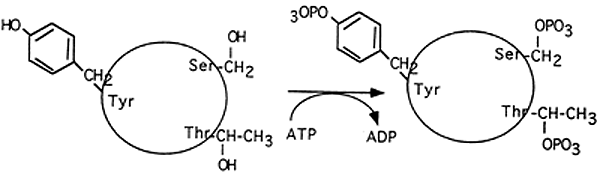
3. Post-translational Modification:
Chemical change in a protein by the covalent attachment of some chemical group to an amino acid.
Phosphorylation (Ser, Thr, Tyr)
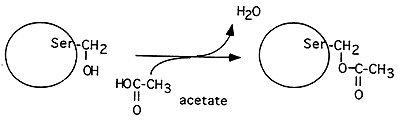
Acylation (attachment of -O=C-CH3)
Hydroxylation (Pro and Lys)
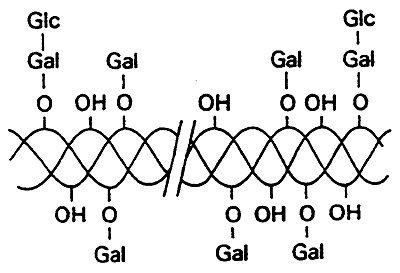
Glycosylation (attachment of sugars)
ADP-Ribosylation (will discuss later)
Carboxylation (attachment of -O2C)
More types of post-translational modification:
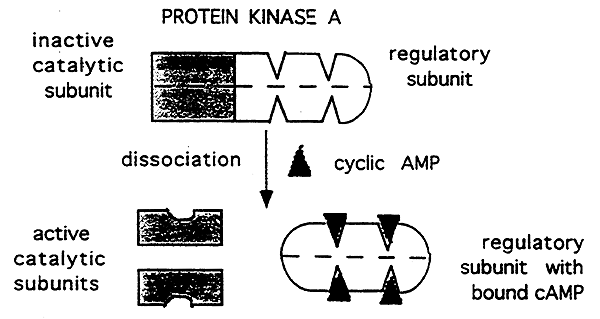
4. Interactions with Control proteins:
Attachment of an inhibitory subunit.
5. Zymogens:
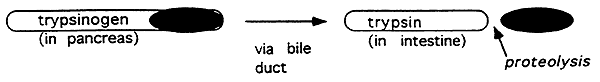
Inactive large precursors of the active form of a protein. The enzymes are activated by removal of a portion of the peptide chain (proteolysis).
© Dr. Noel Sturm 2020